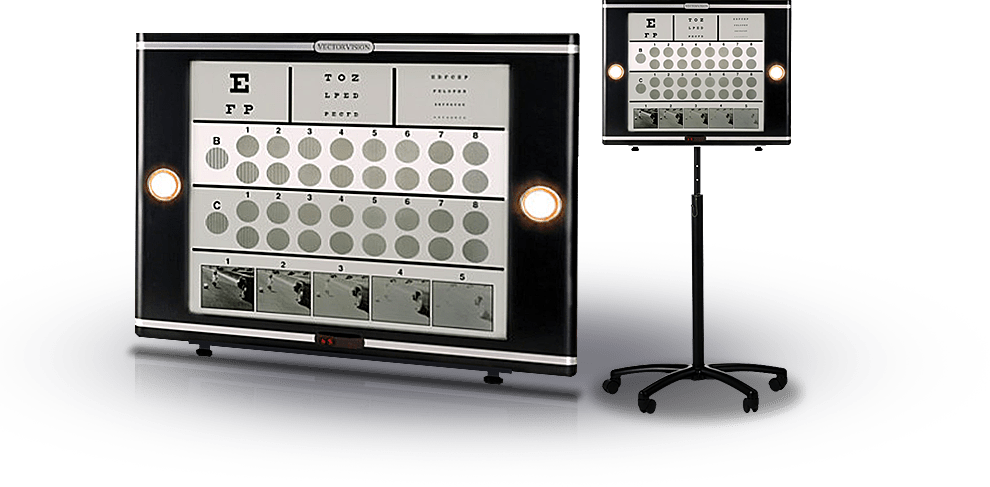
CSV-1000
The CSV-1000 instrument is the recognized Worldwide Leader for standardized contrast sensitivity, ETDRS and glare testing
Why the CSV-1000
The patented auto-calibration circuitry provides for standard and consistent test conditions without a hassle. The instrument automatically controls the test lighting to a level of 85 cd/m2, which is the light level recommended for vision testing by the National Academy of Sciences and adopted by the FDA as the required testing light level for clinical trials. Ease of use is one reason why the CSV-1000 is used in eye clinics in more than 60 countries. All you have to do is turn on the device and it is ready to evaluate patients using standardized and highly accurate tests. The CSV-1000 comes in two forms, the CSV-1000 without glare and the CSV-1000HGT with glare (HGT means Halogen Glare Test). The device is offered with a variety of test faces. These test faces are placed on the front of the CSV-1000 to evaluate contrast sensitivity, ETDRS, low contrast acuity, etc. They are held in position by a magnet and can be easily interchanged. The sister product, the CSV-2000, offers the same range of tests, plus more, via computerized software. No test faces are used with the CSV-2000, the tests are changed via remote control. The different types of test faces available for the CSV-1000 are shown on other pages of this website.
Highlights and benefits
CSV-1000 Features
The CSV-1000 is the first standardized auto-calibrating vision testing instrument. Used in more than 50 FDA clinical trials, 60 countries and 5,000 eye care clinics.
Instrument of Choice
In addition to its use as a clinical tool in more than 5,000 eye care practices world-wide, the CSV-1000 is routinely used for research and to evaluate visual performance. It has been selected for use in numerous multi-center trials in the United States and around the world for the standardized measurement of contrast sensitivity, ETDRS acuity, low contrast acuity and glare sensitivity. These studies include those for the evaluation of LASIK surgery, IOLs, contact lenses, presbyopia correction, athlete visual performance, glaucoma detection and treatment, visual impact of pharmaceutical treatments, nutritional supplements, etc. Companies who have sponsored these trials include Alcon, Abbot Medial Optics, Bausch and Lomb, Merck, Sharp & Dohme, Pharmacia, LensTec, Staar Surgical and Johnson & Johnson to name a few. It has been used by the Olympic Vision Centres to screen athletes for numerous Olympic Games since 1992 and for the evaluation of multiple professional and collegiate sports teams.
More Information About Clinical and Research Use
To learn about how to use the CSV-1000, visit the other pages of this website: for clinical use click here or research use (clinical trials) click here.
The CSV-1000 instrument can be used with a variety of tests. Please visit this page to learn more.
More than 100 published papers have demonstrated the usefulness of the CSV-1000 for a variety of applications including:
- Cataract documentation and evaluation
- Glaucoma screening and treatment optimization
- Sports vision
- Evaluation of retinal disease and the success of treatment
- Measurement of real-world vision for contact lenses, IOLs and refractive surgery technology
- FDA clinical trials
- Post approval (Phase 4) marketing clinical trials

Over the last 20 years, VectorVision testing equipment has become the worldwide benchmark for standardized contrast sensitivity, glare and ETDRS acuity testing.
Read our ReviewsWorking in association with
The World Wide Leader in standardized vision testing








