ESV-3000 Trials
ESV-3000 Widely Used In Clinical Trials

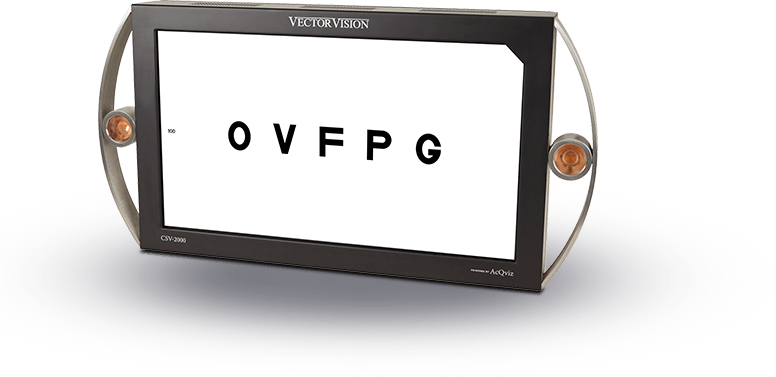
The ESV-3000 was developed in 2009 to provide the same high standards of automatic test light calibration and low maintenance for ETDRS testing as the CSV-2000 and CSV-1000 provide for contrast sensitivity and glare testing. FDA clinical trials typically require that ETDRS testing is conducted with the patient at least 4 meters from the test target to minimize any effect of accommodation. Until the development of the ESV-3000, no standardized ETDRS testing instrument was available to meet this FDA requirement.
The instrument uses light emitting diodes (LEDs) to evenly and continually backlight a large testing surface that provides for testing the full range of ETDRS (20/200 to 20/10) at a distance of 4 meters. The device offers a universal power supply that is compatible with electrical power outlets in every country. Since the device uses solid state LEDs, no bulbs ever have to be replaced and there is no burn-in period. And it has an energy saving footprint! These features make the ESV-3000 a dream for companies who want to conduct clinical trials that use ETDRS acuity, low contrast acuity or letter contrast sensitivity as an outcome measure.
Other Advantages – Multiple Calibrated Testing Light Levels
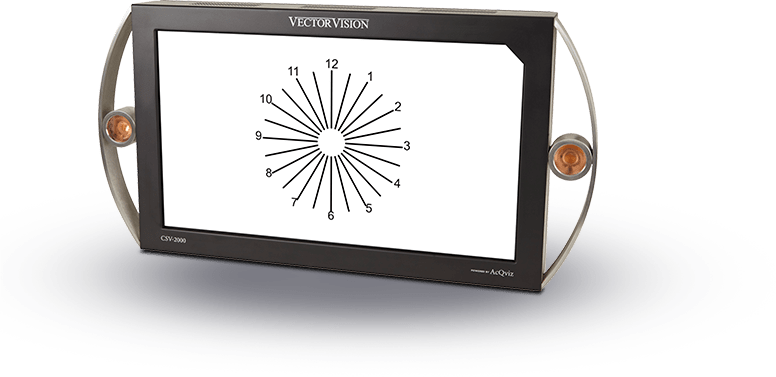
In addition to the standard photopic light level of 85 cd/m2, clinical trial protocols often require patient testing under extreme or varied test lighting conditions. The ESV-3000 can be calibrated to automatically display two test lighting levels, which can easily be switched by the test administration during testing, such as for mesopic testing (3 cd/m2) required in FDA clinical trials. The unique LED backlit system used in the ESV-3000 is capable of providing very high calibrated light levels (super-photopic) and mesopic levels, if needed.
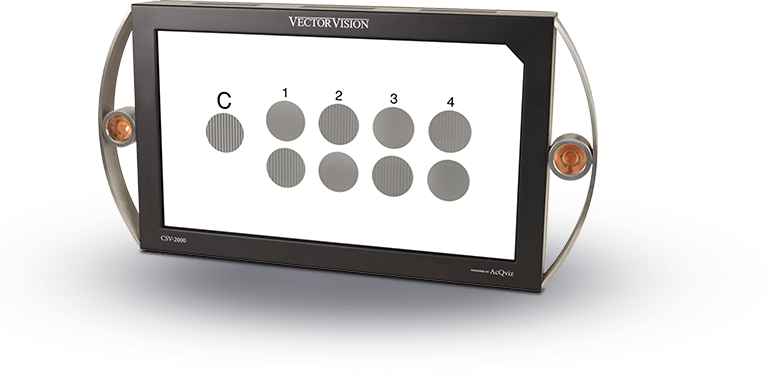
Standardized Letter Contrast Sensitivity for Clinical Trials

The ESV-1000 also offers the only standardized test for letter contrast sensitivity when used in conjunction with the ELCT (Evans Letter Contrast Test). The device provides standardized back-lighting for 16 contrast testing levels ranging from 1% to standard acuity. The letter size is 20/630 on the LogMAR scale at 1 meter.
Success for Clinical Trials
The ESV-3000 has been used in multiple NIH sponsored clinical trials that evaluated optic neuropathies, pharmaceutical company trials that tested ETDRS under standard and very high luminance and AMD treatment trials that tested letter contrast sensitivity.
Featured Product
CSV-2000
CSV-2000 The first and only all-in-one digital vision testing device that offers the full range of vision tests, along with STANDARDIZED contrast sensitivity, glare and visual acuity.
Over the last 20 years, VectorVision testing equipment has become the worldwide benchmark for standardized contrast sensitivity, glare and ETDRS acuity testing.
Read our Reviews